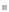Metal-Metal Communication through molecular interfaces in multinuclear titanium complexes
The tris-chelating nitrogen heterocyclic molecule 1,4,5,8,9,12-hexaazatriphenylene (HAT) and its homologues, were often studied in the context of their coordination modes to metal ions, their photophysical properties, liquid crystalline ordering, light-harvesting systems, chirality, and DNA related chemistry. The molecular symmetry of HAT and HATN derivatives leads to especially interesting building blocks creating self-assembled 3D frameworks. Additionally, nonporous network polymers become available from HATN derivatives, including polymer-based hydrogen storage materials. Employing paramagnetic metal centers in HAT and HATN type complexes, magnetic properties of those derivatives, including spin-frustrated behavior or antiferromagnetic couplings are of interest. Electron and energy transfer are ubiquitous in chemical, physical as well as biological processes. In particular, intramolecular electron transfer reactions within polymetallic assemblies are of considerable importance. Starting with the preparation of the Creutz-Taube ion (A) polypyridyl complexes of the d6 metals FeII, RuII, and OsII have received considerable attention, due to their chemical inertness in a variety of oxidation states.
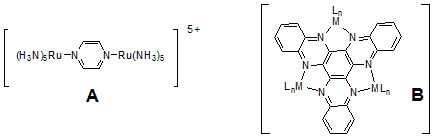
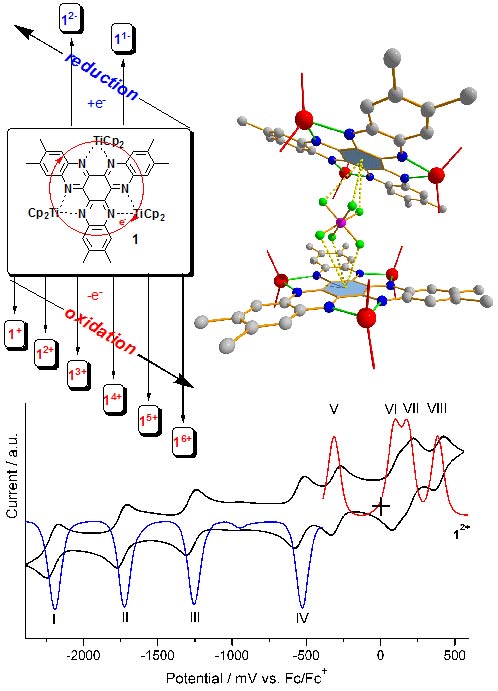
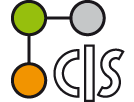
However, the number of comparable HATN derivatives (B) are still low.[1] Often only one or two chelate positions of the HATN ligand are occupied. Herein, we report the synthesis, structure and electrochemical properties of novel cationic trinuclear titanium HATNMe6-complexes, which can be obtained by stepwise oxidation of (Cp2Ti)3(µ3-HATNMe6)(1)[1] derivatives employing ferrocenium salts. The results of the CV and DPV investigations confirmed the structural stability of the redox congeners of 1. Four oxidation waves and four reduction waves were found in the voltammograms of 12+. The stability of the mixed valence states of 1n+ in solution has been determined by calculation of respective comproportionation constants Kc.[2]
[1] I. M. Piglosiewicz, R. Beckhaus, W. Saak, D. Haase, J. Am. Chem. Soc. 2005, 127, 14190-14191.
[2] I. M. Piglosiewicz, R. Beckhaus, G. Wittstock, W. Saak, D. Haase, Inorg. Chem. 2007, 46, 7610-7620.
Figure below:
(Cp2Ti)3(µ3-HATNMe6) and ist cyclovoltammogram exhibiting four oxidation and four reduction waves