Heterogeneous catalysis with gold
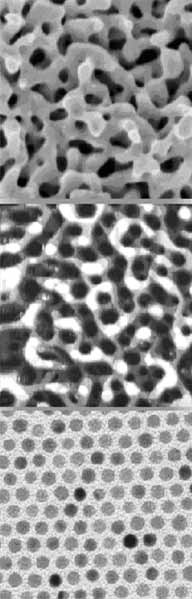
Gold was long considered to be a catalytically inactive metal until recently, when small nano-size gold particles were found to catalyze various reactions, including CO oxidation, NO reduction, water-gas shift reaction, and hydrocarbon oxidations including propylene epoxidation with high selectivity (>90%). Gold catalysts are promising candidates to improve automotive emissions control, particularly the cold-startup problem because they show significant catalytic activity at much lower temperatures than the catalysts currently used. Active gold catalysts studied so far are composed of two main components: nano-scale gold particles and a metal oxide support. Some researchers have proposed that the catalytic activity originates from gold itself and that the oxide support is a passive supporting material, which provides nucleation and growth sites for gold nano-particles. In these models gold atoms with a low coordination number or modification of the electronic properties of the gold particles due to quantum confinement have been attributed to the origin of the catalytic activity of gold. Others emphasize the roles of the oxide support including (1) transferring charge from or to the oxide support to the gold particle, (2) providing adsorption sites for reactants, and (3) providing reactive sites at the gold-oxide interface. However, a clear hierarchy of model systems in combination with a strong theoretical backup has been missing so far. CIS members Bäumer and Al-Shamery tried to overcome this situation by preparing different well-defined Au catalysts and studying CO oxidation on the various systems. [1-4,6]. Nanoporous gold, for instance, was prepared by sputtering a Au(111) single crystal surface [1], as well as by dealloying of silver-gold alloys. Both systems exhibit an open sponge-like morphology of interconnecting ligaments on the nanometer length scale [2,3,7]. While with the first system the adsorption and reaction behaviour of such nanoporous Au materials could be studied under UHV conditions, the latter one allowed to carry out catalytic experiments under ambient pressures. These supportless catalysts were compared with well-defined Au nanoparticles either prepared by plasma etching of colloidal particles self-assembled on suitable supports or Au particles vapour deposited on HOPG [4]. As an example, the studies revealed that adsorption of CO takes place at two different sites of undercoordinated gold atoms in a universal manner. [5+6].
Figure: Top: Scanning tunneling micrograph of oxygen-sputtered Au(111) Middle: Scanning electron micrograph of nanoporous gold foam from dealloying gold-silver alloy Bottom: Transmission electron microscopical image of gold colloids of 6 nm diameter capped with dodecane thiol ligands
In order to obtain a microscopic interpretation of the experimental results the Theoretical Chemistry group of CIS (Klüner) performed large scale DFT-calculations. The p(nx1; n=1-8) Au(332) was used as a model surface exhibiting low coordinated adsorption sites for CO. The results of the Klüner group clearly confirm that the CO-moldecule is located at steps and kinks and not on terrace sites [6]. Thus, a detailed mechanistic understanding could be achieved which paves the way for a rational design of new catalysts based on first principles. Another important result with respect to the catalytic properties was that catalytic activity of Au can not only be expected for nanoparticles in the nm regime but also for supportless nanoporous systems. In first experiments, high conversions for CO oxidation could be obtained even at -20°C [2].
[1] J. Biener, M.M. Biener, T. Nowitzki, A.V. Hamza, C.M. Friend, V. Zielasek, M. Bäumer, ChemPhysChem 7 (2006) 1906.
[2] V. Zielasek, B. Jürgens, Ch. Schulz, J. Biener, M.M. Biener, A.V. Hamza, M. Bäumer, Angew. Chem., Int. Ed. 45 (2006) 8241.
[3] B. Jürgens, Ch. Kübel, Ch. Schulz, T. Nowitzki, V. Zielasek, J. Biener, M.M. Biener, A.V. Hamza, M. Bäumer, Gold Bull., in press.
[4] T. Matsumoto, P. Nickut, H. Tsunoyama, K. Watanabe, T. Tsukuda, K. Al-Shamery, Y. Matsumoto, Surf. Sci., in press
[5] T. Nowitzki, P. Nickut, C. Deiter, J. Wollschläger, K. Al-Shamery, M. Bäumer, Surf. Sci. 600 (2006) 3595.
[6] W.-L. Yim, T. Nowitzki, M. Necke, H. Schnars, P. Nickut, J. Biener, M.M. Biener, V. Zielasek, K. Al-Shamery, T. Klüner, M. Bäumer, J. Phys. Chem. C 111 (2007) 445.
Patent
[7] M. Bäumer, J. Biener, M. Biener, A. Hamza, B. Jürgens, Ch. Schulz, Patent pending;