New colloid based Pt-catalysts
Katharina Al-Shamery, Marcus Bäumer, Holger Borchert, Thorsten Klüner, Joana Kólny und Jürgen Parisi
Supported metals have a wide range of applications in heterogeneous catalysis and particle size and shape is one of the key factors influencing activity and selectivity. The classical ways of preparing supported catalysts are impregnation or precipitation techniques allowing only poor control in this respect. A promising issue for improvement is the use of metal nanoparticles prepared by colloidal chemistry which allows obtaining particles with well-defined size and shape by the use of stabilizing ligands in the synthesis.
Even beyond advanced control of particle sizes and morphology, the approach of colloidal chemistry introduces also the new possibility to prepare supported catalysts where the metal surface is partly covered by ligand molecules. This opens completely new perspectives to influence on activity and selectivity due to electronic and sterical effects.
In this context, first efforts were undertaken within the CIS to elucidate systematically the potential of colloidally prepared, ligand-capped metal nanoparticles for applications in heterogeneous catalysis. Therefore, Pt nanoparticles capped with different ligands (alkanethiols and alkylamines) were prepared by colloidal chemistry and deposited on oxide supports. The supported nanoparticles were investigated with respect to CO oxidation as a first, simple test reaction. Experiments with in situ FTIR spectroscopy provided clear evidence that small molecules like CO can pass the ligand shell and adsorb on free surface sites. Furthermore, activity for CO oxidation could be observed in the presence of the organic ligands, in some cases at temperatures from ~160-180°C on [1].
From these first positive results arises a high application potential for more complex reactions in heterogeneous catalysis where one can intend to control selectivities by ligand-induced effects. This issue will be addressed in future work.
References:
[1] H. Borchert, D. Fenske, J. Kolny-Olesiak, J. Parisi, K. Al-Shamery, and M. Bäumer, Angew. Chem. 119, 2981 (2007); Angew. Chem. Int. Ed. 46, 2923 (2007).
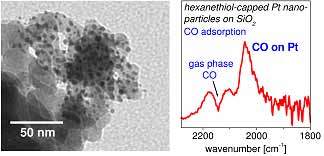
Figure:
Left: TEM-image of colloidal Pt-particles on a silica support after catalytic CO-oxidation reaction at
473 K
Right: Drift-spectrum of CO-adsorption of hexanethiol capped Pt-particles